Novel Kinase Inhibitory Aplithianines
Summary
The National Cancer Institute (NCI) seeks research co-development partners and/or licensees for a class of novel aplithianine-derived small molecule analogs that compete with ATP for binding on a range of clinically relevant kinases including:
- Oncogenic gene fusion DNAJB1-PRKACA (PKADJ)
- Wild type protein kinase A (PKA)
- Protein kinase G (PKG)
- Ccdc2-like kinases (CLK) 1 & 2
- DYRK family of kinases
Description of Technology
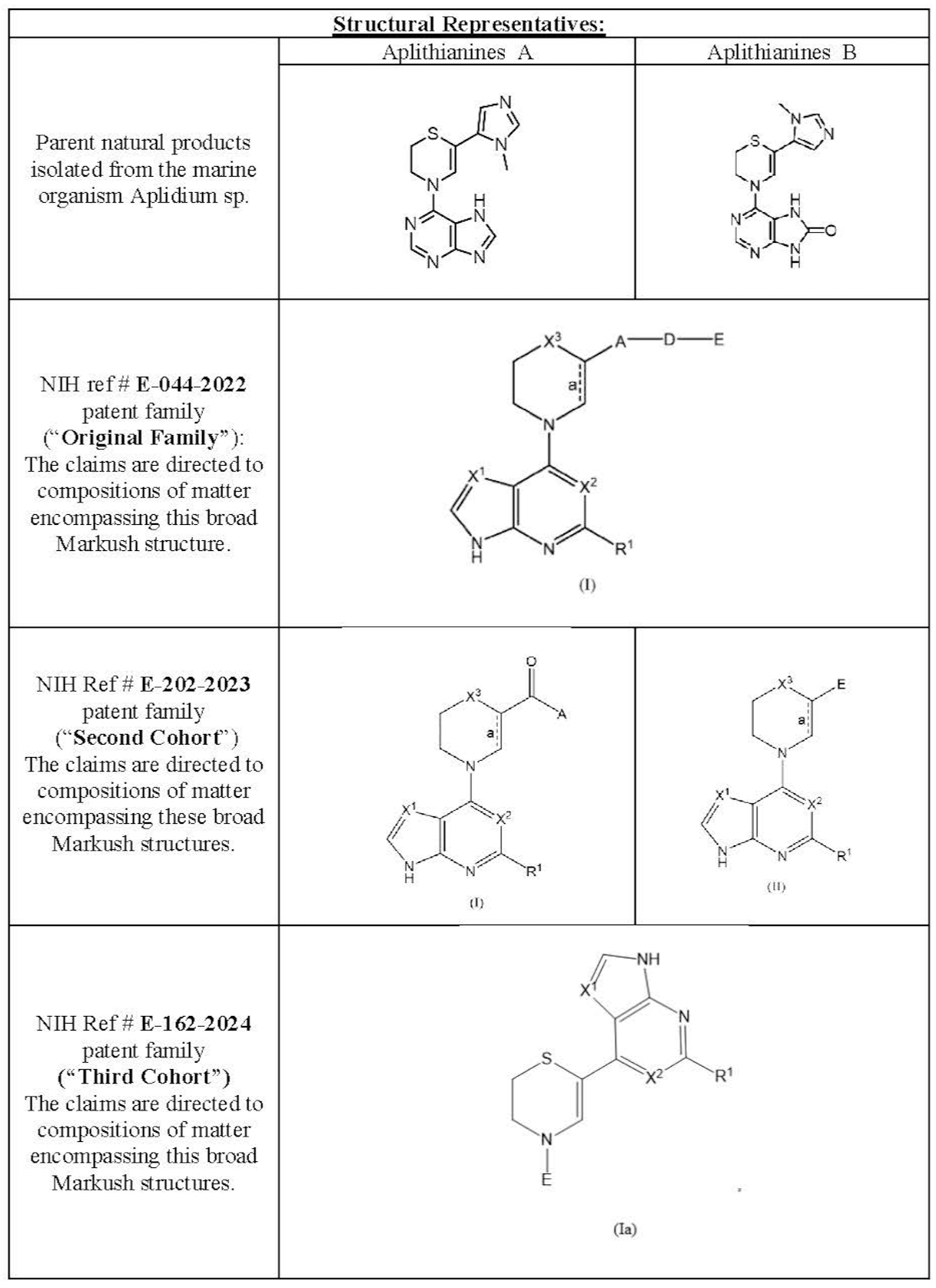
In 2022, the NCI Molecular Targets Program (MTP) completed a screen of ~150,000 pre-fractionated natural products from the NCI Program for Natural Product Discovery (NPNPD). From this screen, a class of active compounds, named Aplithianines A & B (isolated from the marine organism Aplidium sp.), showed broad potential applicability to numerous kinases of importance including but not limited to:
- Oncogenic gene fusion DNAJB1-PRKACA (PKADJ)
- Implicated in an ultra-rare adolescent liver cancer
- Wild type protein kinase A (PKA)
- Implicated in Cushing’s Disease
- Protein kinase G (PKG)
- Potential treatment of malaria
- Ccdc2-like kinases (CLK) 1 & 2
- Implicated in gastric cancer
- DYRK family of kinases
- Implicated in gastric or colon cancer as well as infections caused by a protozoa or parasites
This Technology describes the Original Family of compounds filed. Subsequent to this filing, two additional cohorts of related, but patentably distinct Cohorts of compounds have been filed under NIH Ref # E-202-2023 and E-164-2024. Both the Second and the Third Cohorts comprise the same chemical scaffold of the broadest generic formula of this Original Family but represent patentably distinct subgenus formulas.
The specificity of several of the compounds have been examined in kinase panels to demonstrate that while applicable to a range of kinases, they are not promiscuous kinase inhibitors. The subject kinase inhibitors have broad potential commercial applicability’s for cancer, immune suppression, preventing organ rejection, treating diabetic neuropathic pain, malaria, or protozoa infection. To date there are no approved therapeutics targeting DNAJB1-PRKCA, an oncogenic gene fusion is ubiquitously and exclusively detected in the tumors of patients with ultra-rare fibrolamellar hepatocellular carcinoma FLHCC.
The NCI seeks licensing and/or co-development research collaborations for the future development of Kinase Inhibitory Aplithianines targeting DNAJB1-PRKACA (PKADJ), PKA, PKG, CLK, and/or DYRK.
Potential Commercial Applications
• Gastric cancer
• Ultra-rare adolescent liver cancer
• Solid cancers susceptible to kinase inhibitors
• Cushing’s Disease
• Transplantation
• Diabetic neuropathic pain
• Malaria
• Protozoa infection
Competitive Advantages
• Applicability to numerous clinically relevant kinases, including:
- Oncogenic gene fusion DNAJB1-PRKACA (PKADJ)
- Wild type protein kinase A (PKA)
- Protein kinase G (PKG)
- Ccdc2-like kinases (CLK) 1 & 2
- DYRK family of kinases
• Applicable to a range of kinases, but are not promiscuous kinase inhibitors
• Broad potential commercial applicability for several blockbuster indications including: cancer, immune suppression, transplantation, diabetic neuropathic pain, malaria, and protozoa infection
• No approved therapeutics targeting DNAJB1-PRKCA
