Design and Biological Activity of Novel Stealth Polymeric Lipid Nanoparticles for Enhanced Delivery of Hydrophobic Photodynamic Therapy Drugs
Nanoparticles such as lipid-based nanoparticles (LNPs) represent a relatively new era of targeted drug delivery systems wherein these biocompatible particles can carry the drug(s) of interest to a specific tumor site. The new generation of nanoparticles, known as stealth nanoparticles, are engineered to have a coating of polyethylene glycol polymer (PEG) or other glycolipids that enable them to evade the immune system and have a longer circulation lifespan as well as improved bioavailability to diseased tissue and reduced non-specific toxicity.
Scientists at the National Cancer Institute (NCI) have developed the formulation of novel stealth nanoparticles that have high stability and high payload capacity. These nanoparticles are vesicles made of binary lipid bilayer comprising phospholipid DC8,9PC and a PEGylated lipid such as DSPE-PEG2000. The vesicles can carry high amounts of payload of a hydrophobic photodynamic drug (PDT) such as HPPH (up to 0.5mg of HPPH/mg lipid), a photosensitive drug that has anti-cancer properties when irradiated by near-infrared light. These HPPH- loaded vesicles exhibit >90% serum stability and are stable for at least 42 days at room temperature. These vesicles showed higher doses of the HPPH in the blood samples of intravenously injected mice based on their pharmacokinetic profile (monitored up to 264 hours).
When tested in-vivo in animal models, they demonstrate high LNP-associated HPPH uptake in HT29 mice tumors due to improved tumor accumulation and show better tumor regression in Colon-26 bearing BALB/c mouse model with no tumor recurrence up to 100 days compared with animal survival data observed with Tween 80- HPPH , the nanoparticle formulation used in current clinical trials. These HPPH-loaded vesicles represent a novel targeted anti-cancer therapeutic that can also be engineered to carry other cytotoxic, imaging, nucleic acid agents for targeted therapy/diagnostics of other disease types.
Competitive Advantages:
- Superior stealth formulation (i.e. evasion from immune recognition) due to high PEGylation ability of the nanoparticle - important for in vivo applications
- High payload of PDT drug HPPH: up to 0.5mg of HPPH/mg lipid
- Vesicles remain stable for at least 42 days upon storage at room temperature – potential translational (See Figure 1) advantage. Improved stability of vesicles compared to the vesicles published previously by the same group
- Enhanced tumor uptake of LNP-associated HPPH in HT29 mice due to increased PEG density on the surface of the liposomes
- Superior tumor regression efficiencies in Colon-26 mouse model compared to the nano-formulation of Tween 80-HPPH, which is used in clinical trials
- Prolonged circulation lifetime of payload
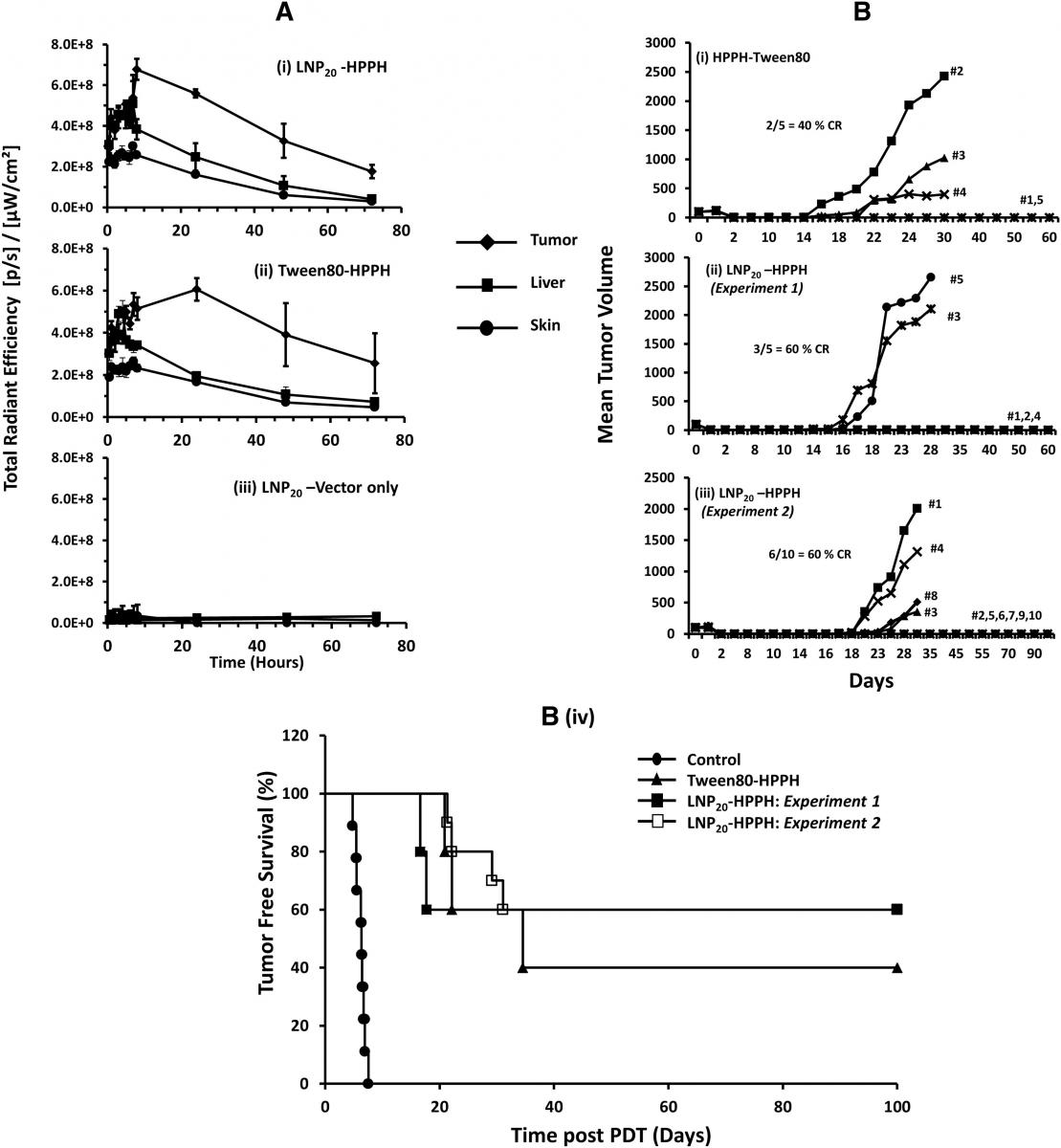
Figure 1. (A) In vivo tissue distribution of LNP formulated HPPH in liver, tumor and skin of tumor-bearing mice by optical imaging: CT-26 tumor-bearing BALB/c mice were intravenously injected (groups of five) using either Tween 80-HPPH or LNP20-HPPH. LNP20 without HPPH were used to obtain background signals. Images were collected at various time intervals and average radiant efficiency of HPPH in tumor, liver and skin was determined. (B) In Vivo PDT response and antitumor activity of LNP20-HPPH in CT-26 bearing BALB/c mice: Tween 80-HPPH or LNP20-HPPH were intravenously injected in tumor-bearing mice at 0.47μmol HPPH/kg body weight. Laser treatments were done post 4 h for LNP20-HPPH-injected animals and post 24 h injections of Tween 80-HPPH. Tumor volumes were measured at indicated days. (B (i) & (ii)) Tween80-HPPH and LNP20-HPPH respectively (five mice per set). (B (iii)) LNP20-HPPH injected mice (group of ten animals). (B (iv)) Animal survival data are presented as Kaplan Meier graph comparing LNP20-HPPH (two independent experiments) and Tween 80-HPPH.
Commercial Applications:
- Nanoparticle platform loaded with PDT and non-PDT drugs for delivery of a therapeutic drug, gene therapy, or imagining agent for the treatment or diagnosis of disease.
- Drugs otherwise demonstrating inability to reach target sites (which contributes to exceptionally high attrition rates of new chemical entities [NCEs] across all therapeutic areas, with <15% of drugs gaining approval by regulatory authorities).
