Efficacious Fluorinated Cytidine Analog Cancer Therapeutic With Low Toxicity In Animal Studies
Cytidine analogs remain an area of active drug discovery and development, with five FDA approved drugs for the treatment of acute myeloid leukemia (AML). Two of these drugs, azacitidine (Vidaza®) and decitabine (Dacogen®), which were approved for myelodysplastic syndromes in 2004 and 2006, respectively, inhibit the DNA maintenance methyltransferase DNMT1. Because of the general toxicity of azacitidines, other nucleoside analogs are favored as therapeutics.
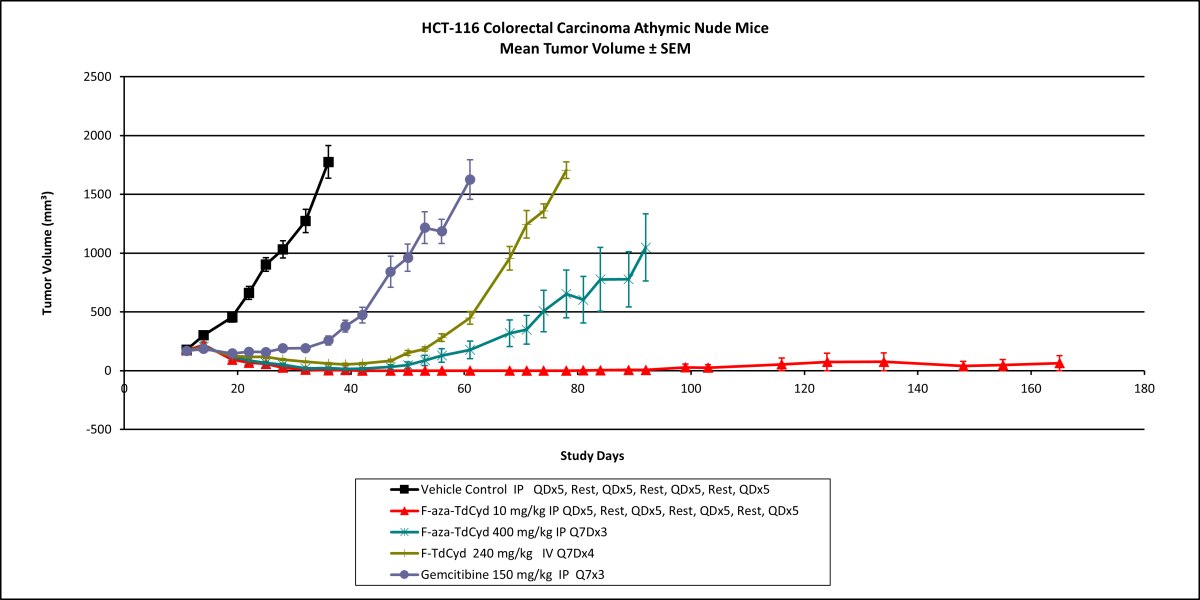
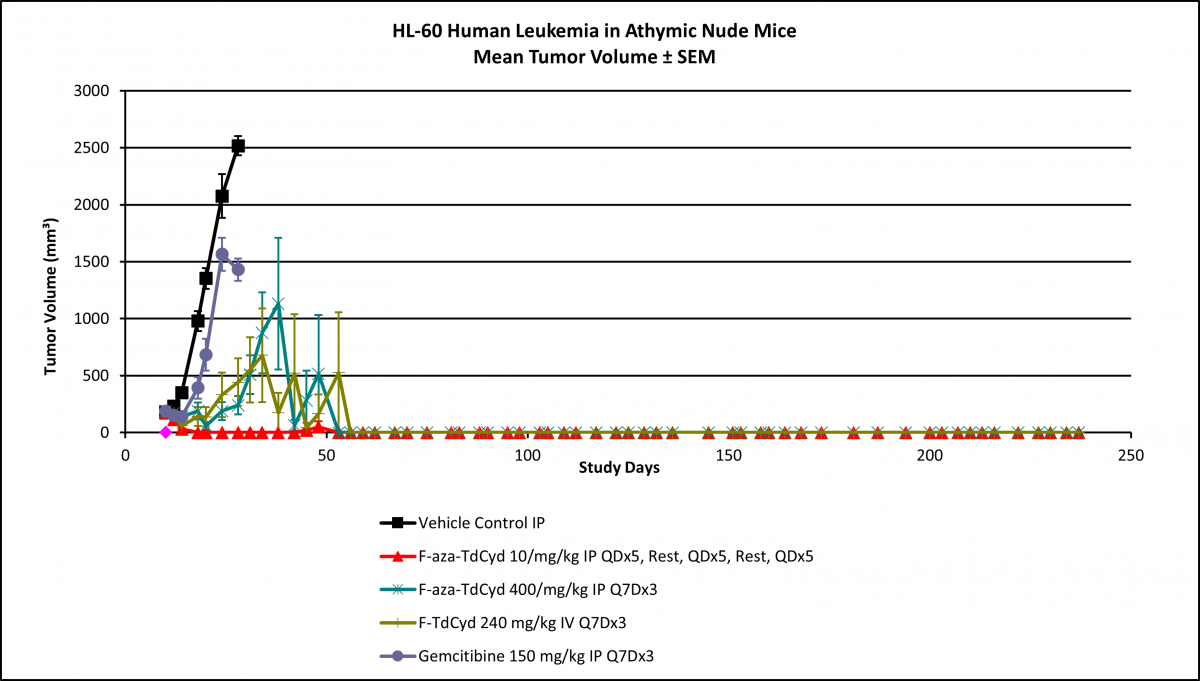
Researchers in the National Cancer Institute’s Drug Synthesis and Chemistry Branch recently synthesized a fluorinated cytidine and demonstrated that incorporation of a fluorine atom into the chemical structure of cytidine derivatives is effective in producing changes in target potency, selectivity, and overall toxicity. A comparative in vivo efficacy study with gemcitabine in several human tumor xenograft studies indicated low toxicity and high efficacy. In colon cancer mouse models, the fluorinated cytidine produced complete regression of the tumors in all studied mice with a response that proved durable beyond post-implant day 150. Similarly, complete tumor regression was observed in a leukemia xenograft, shown below.
Competitive Advantages:
- Oral bioavailability
- Incorporating fluorine increases lipophilicity, through steric and electronic effects, can block metabolism and increase potency
- Increased selectivity and decreased toxicity compared to other aza-cytidines.
Commercial Applications:
- Potential therapeutic for various types of cancers including colon cancer and leukemia
