A Preclinical Orthotopic Model for Glioblastoma Multiforme that Represents Key Pathways Aberrant in Human Brain Cancer
Current therapies for glioblastoma multiforme (GBM), the highest grade malignant brain tumor, are mostly ineffective, and better preclinical model systems are needed to increase the successful translation of drug discovery efforts into the clinic. Scientists at the National Cancer Institute (NCI) have developed and characterized an orthotopic genetically engineered mouse (GEM)-derived model of GBM that closely recapitulates various human GBM subtypes and is useful for preclinical evaluation of candidate therapeutics. The GEM-derived GBM model harbors perturbations in the receptor tyrosine kinase (RTK), phosphoinositide 3-kinase (PI3K) and retinoblastoma (RB) tumor suppressor networks and develops spontaneous p53 aberrations upon induction of the constitutively active mutant KRASG12D and deletion of phosphatase and tensin homolog (PTEN) alleles; the orthotopically implanted mice are referred to as ‘TRP’ mice. The TRP mice develop high grade GBM that are histologically similar to human GBM within weeks allowing the creation of large preclinical cohorts that are tractable for therapeutic evaluation.
Figure: Orthotopic GBM model characterization
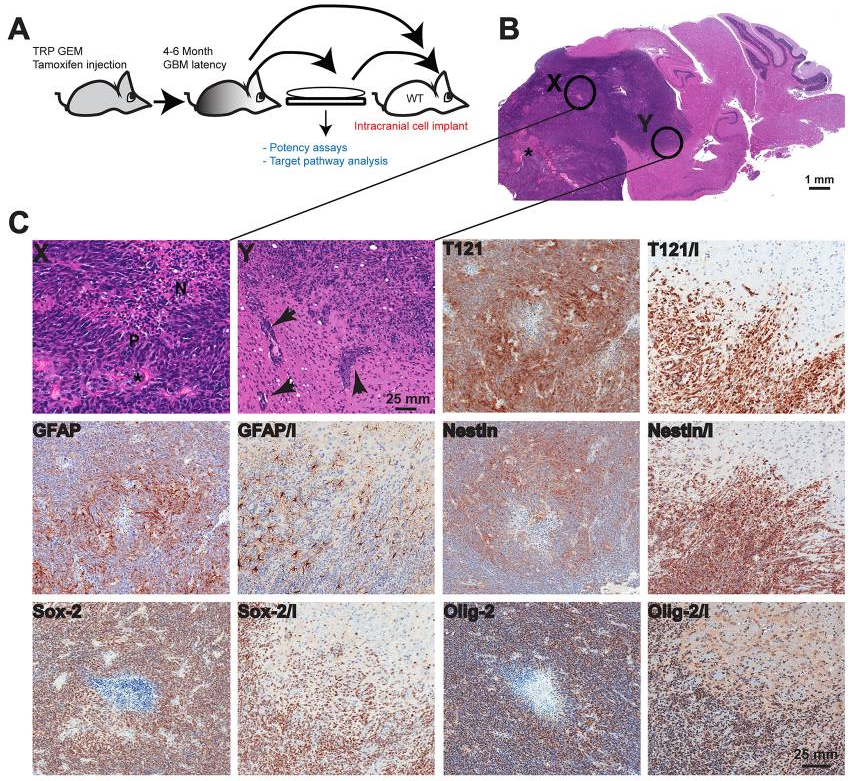
(A) Tumor cells were isolated from TRP grade IV astrocytoma and cultured for several passages prior to intracranial injection into syngeneic mouse brains, or cells were implanted directly. (B) Both methods resulted in grade IV astrocyomas. Orthotopic tumors have large numbers of variably sized irregular blood vessels (*). Enlarged images of areas labeled X and Y are shown in panel C. (C) Orthotopic GBMs feature necrotic foci (N in region labeled X) in central regions that are lined by pseudopalisading tumor cells (P). The invasive tumor cells often track along adjacent small blood vessels (arrows in region labeled Y), as well as diffusely invading through the recipient’s neuropil. Two images are shown for each marker stain; in the second, invasion is indicated by ‘I’ where orthotopic tumors contain foci of extensive invasion into the adjacent recipient normal brain. Tumor cells express variable levels of T121 with increased levels at the invasive edge highlighting individual invading tumor cells (T121 and T121/I). GFAP expression is heterogeneous with greater numbers of negative cells at the periphery (GFAP and GFAP/I). Nestin expression is also greater at the invasive front (Nestin and Nestin/I). Most tumor cells express Olig-2 (Olig-2 and Olig-2/I) and Sox-2 (Sox-2 and Sox-2/I).
Competitive Advantages:
- Rapid preclinical evaluation of therapeutics. Primary cells from the GEM-GBM injected orthotopically into immune competent syngeneic mice brains induce grade IV tumors within 2 to 3 weeks.
- The GEM-GBM model represents a range of GBM cell types, recapitulating the heterogeneous cell population found in human GBM.
- GBM tumors are distinct and measurable on MRI scans and DCE-MRI properties have been described
- Superior to human cell xenograft models: syngeneic immunocompetent model allows for the evaluation of checkpoint inhibitors and other immunotherapeutic drugs.
Commercial Applications:
- Tractable mouse model of human glioblastoma multiforme for preclinical evaluation of GBM therapies
