Treating Kidney Disorders and Diabetic Nephropathy with N-acetyl mannosamine (ManNAc)
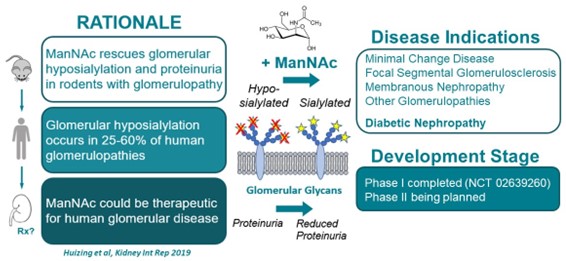
N-acetylmannosamine (ManNAc) is a small uncharged physiological molecule that crosses membranes readily and is the natural precursor of intracellular sialic acid synthesis. NHGRI investigators discovered that ManNAc can be used for therapeutic purposes, including treating certain kidney diseases (e.g., those involving proteinuria and hematuria), resulting primarily or secondarily from hyposialylation (lack of sialic acid). Notably, ManNAc can also be used to treat diabetic nephropathy or diabetes.
ManNAc therapy is given orally and shows long-term safety and biochemical efficacy, consistent with its mechanism of action in human.
Thus, the following fields of use are available for licensing: Treating kidney disorders due to hyposialylation of the glomerulae or sialic acid deficiency, including but not limited to minimal change disease glomerulopathy, focal segmental glomerulosclerosis, and membranous nephropathy in humans with oral formulations of N-acetyl mannosamine (ManNAc) or derivative. Treating diabetic nephropathy or diabetes are also available.
- ManNAc is easy to administer to patients (oral administration).
- Long-term ManNAc administration has been shown to be safe and well-tolerated in humans.
- There are many issued patents in the U.S, Canada, Europe, Japan, and Israel for this indication.
- Extensive published and unpublished preclinical data for the kidney indication is available.
- A Phase I clinical trial of ManNAc in subjects with primary glomerular diseases has been completed, and Phase II study in subjects with primary focal segmental glomerulosclerosis has been approved by NIH IRB.
