Reporter Assay for Detection and Quantitation of Replication-Competent Gammaretrovirus
Gammaretroviral vectors were the first viral gene-therapy vectors to enter clinical trials and remain in use. One potential hazard associated with the use of such vectors is the presence of replication-competent retroviruses (RCR) in the vector preparations – either as a result of: 1) recombination events between the plasmids used for vector production, 2) interactions between the plasmids and endogenous retroviral sequences in the packaging cell lines, or 3) as a result of contamination in the laboratory. RCRs are potentially pathogenic and shown to induce malignancy in mice and non-human primates. Therefore, it is critical that vector preparations be rigorously tested to exclude the presence of RCRs given that the Food and Drug Administration (FDA) requires all vector preparations be screened for RCRs.
At present, the FDA recommends RCR testing by inoculating an aliquot of each vector preparation into a permissive cell line and passaging the cells for at least three weeks (i.e., amplification phase). Cell-free media from the amplification phase can then be analyzed by a variety of methods to demonstrate the presence or absence of RCRs.
Scientists at the National Cancer Institute (NCI) have developed a new research method for the detection of RCRs in gammaretrovirus-based gene-therapy products and the cell lines used to make them. The assay uses one of a series of DERSE (Detector of Exogenous Retroviral Sequence Elements) plasmids, stably maintained in a cell line permissive for the retrovirus of interest. Utilizing the unique reverse-transcription step of the retroviral infection cycle, a functional reporter gene is formed in the DERSE cell culture only after infection with an RCR. The DERSE assay can be constructed, potentially, to detect any RCR and can use any reporter gene.
This invention is available for licensing.
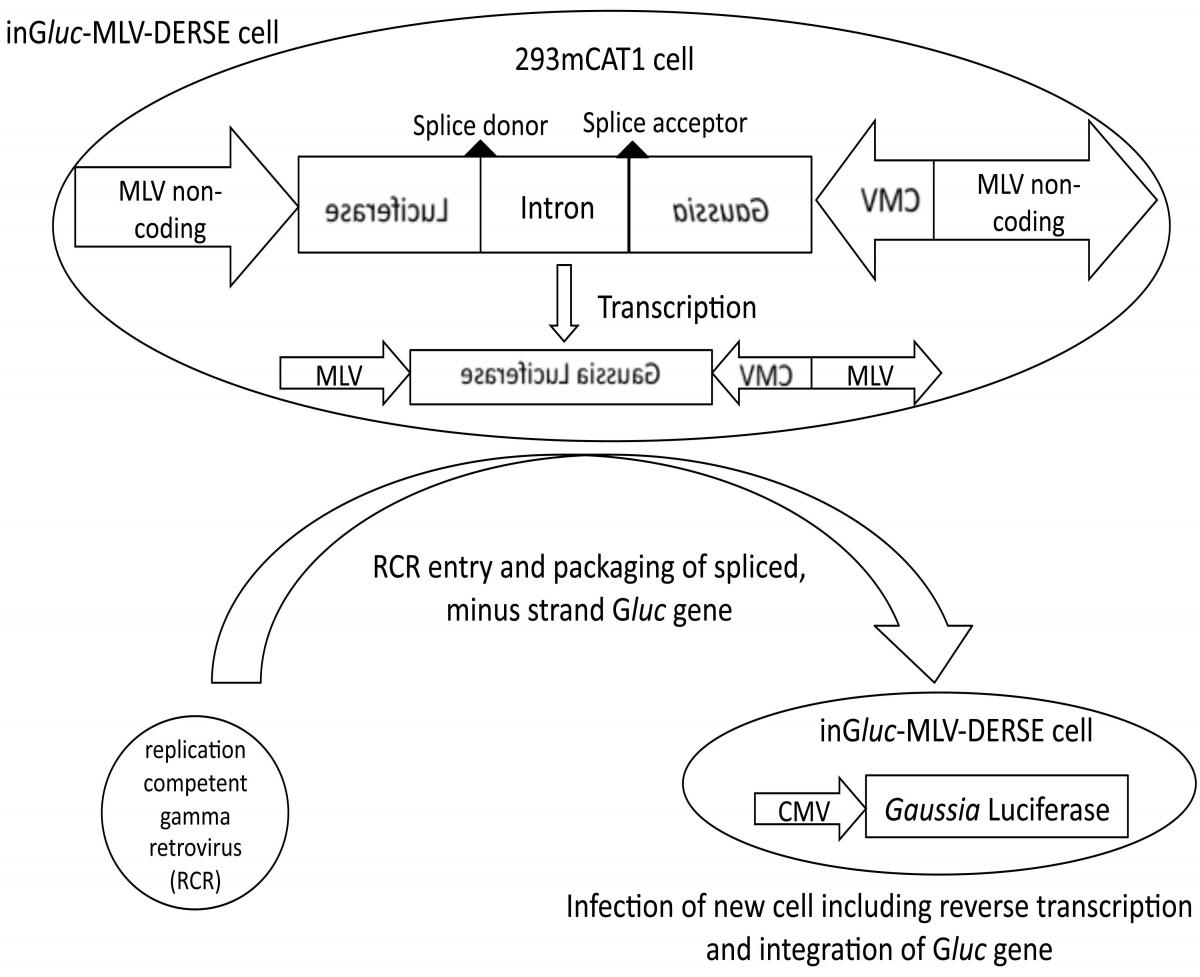
Figure: Schematic of inGluc-MLV-DERSE assay
The inGluc-MLV-DERSE plasmid consists of a Gaussia Luciferase (Gluc) sequence oriented in a reverse direction with respect to flanking MLV non-coding sequences. Within the non-coding Gluc sequence is an intron that is oriented in a forward direction relative to the viral non-coding regions (and can be spliced by the host cell). The plasmid is maintained in 293mCAT1 cells. In the absence of RCR, only minus-strand, spliced Gluc sequences are present in the cell. An RCR that infects the DERSE cell can package the RNA containing the minus-strand Gluc sequence. In the next round of infection reverse transcription of the encapsidated RNA produces a double-stranded DNA containing an uninterrupted Gluc gene. This gene is an intact, coding Gluc sequence that is subsequently integrated into the DNA of, and expressed by, the newly infected cell. Expression of Gluc is under the control of the cytomegalovirus (CMV) promoter.
Competitive Advantages:
- Provides reliable results in roughly half the time of traditional methods for detecting replication-competent retrovirus (RCR)
- Overcomes the inhibitory effect of excess gene-therapy vector in the detection of RCRs
- Does not require the identification of foci
- Useful for screening both gene-therapy products and the packaging cell lines used for vector production
- Enhanced detection sensitivity as the indicating reporter is only formed in cells infected with replication-competent retrovirus
Commercial Applications:
- Laboratory tool to investigate molecular mechanisms of retroviral replication
- Research tool to screen gene therapy regimens
- Manufacturing tool to screen viral vector preparations
