Monomeric and Oligomeric Compounds as Contraceptives and Endocrine Therapeutics
The options for male contraceptives are limited. Research is ongoing to develop a male contraceptive based on hormonal activity. Testosterone is one of the hormones necessary in producing sperm. Testosterone is absolutely required as a hormone for male fertility. Derivatives of testosterone for male contraceptives currently in clinical trials are associated with estrogenic deficiency. This deficiency can cause several issues including, but not limited to, bone density loss, risk of obesity, cardiovascular disease, and/or ineffective carbohydrate or lipid metabolism.
Researchers at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) have developed a new chemical entity and related embodiments of the following formula:
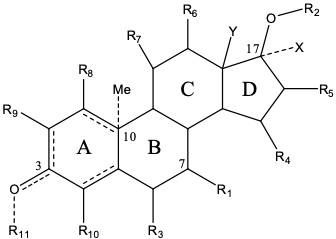
Formula 1. This invention discloses embodiments of the above compound, or a pharmaceutically acceptable salt, a prodrug, a solvate, or a tautomer thereof.
Disclosed monomeric compounds and embodiments exhibit androgenic activity. Disclosed oligomeric compounds can provide control of receptor activation or deactivation and/or treatment through the coupling of steroidal-based compounds to one another or with therapeutic agents. Depending on the type and length between the compounds that form the oligomer, the rate at which each component of the oligomer release can be controlled as in ‘time release’. The appropriate dosage would depend on a variety of factors defined during further development.
NICHD is seeking research co-development partners and/or licensees for development of this invention as a male contraceptive.
Competitive Advantages:
- Significant, unmet medical need given few current options for male contraceptives
- Timed release of active ingredients
Commercial Applications:
- Contraceptive use by humans, particularly male, and animals
- Compounds used as endocrine therapy
